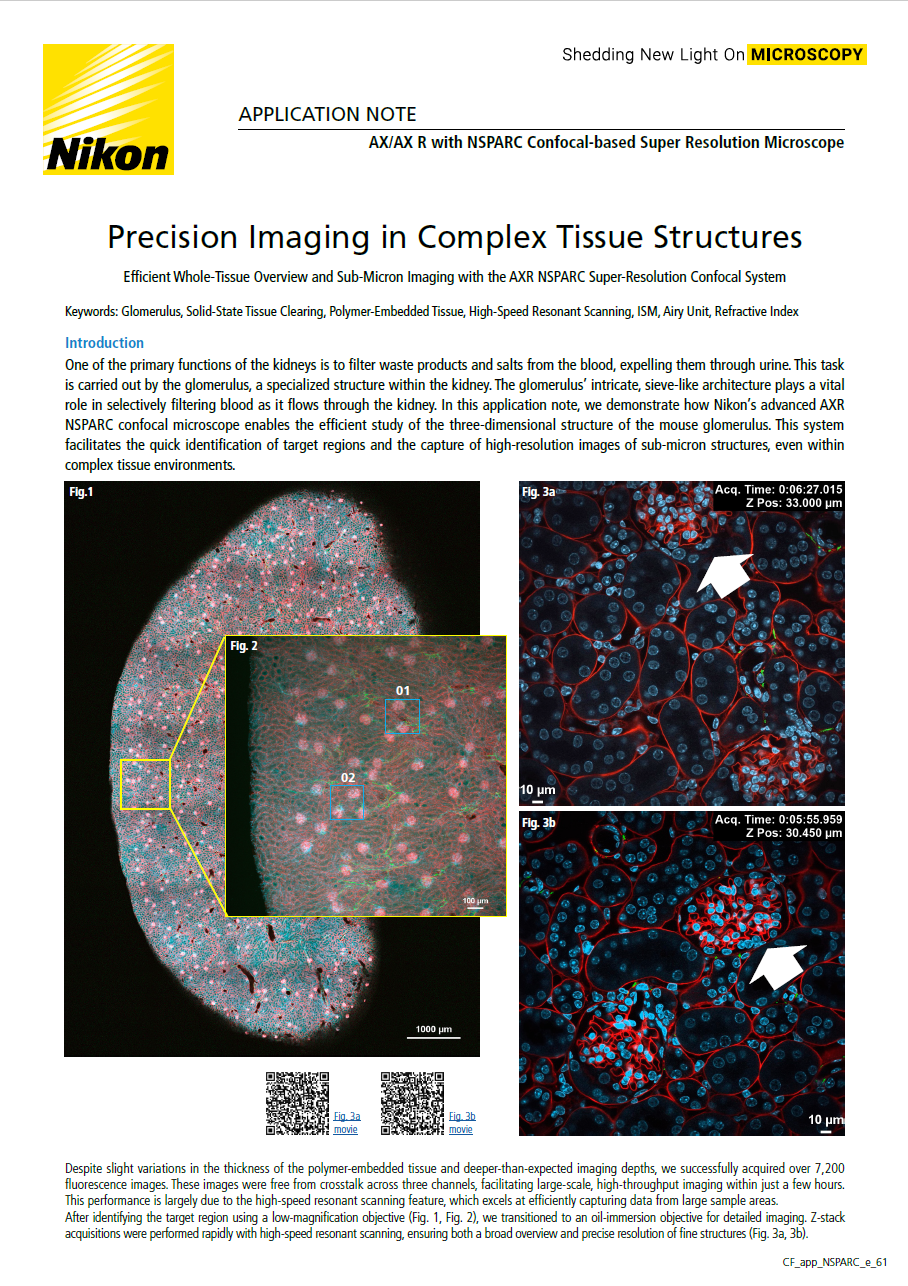
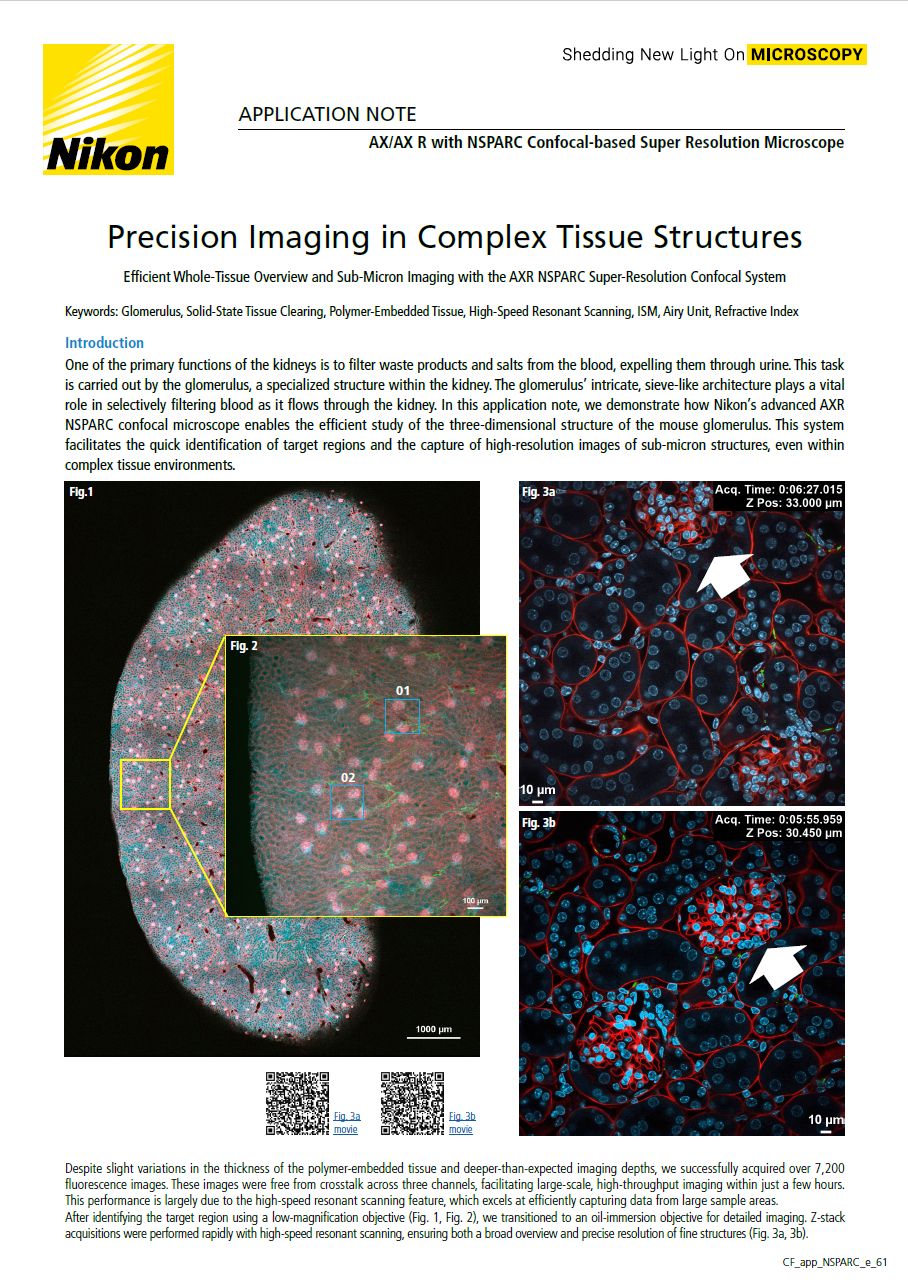
One of the primary functions of the kidneys is to filter waste products and salts from the blood, expelling them through urine. This task is carried out by the glomerulus, a specialized structure within the kidney. The glomerulus’ intricate, sieve-like architecture plays a vital role in selectively filtering blood as it flows through the kidney. In this application note, we demonstrate how Nikon’s advanced AXR NSPARC confocal microscope enables the efficient study of the three-dimensional structure of the mouse glomerulus. This system facilitates the quick identification of target regions and the capture of high-resolution images of sub-micron structures, even within complex tissue environments.
Keywords: Glomerulus, Solid-State Tissue Clearing, Polymer-Embedded Tissue, High-Speed Resonant Scanning, ISM, Airy Unit, Refractive Index
Despite slight variations in the thickness of the polymer-embedded tissue and deeper-than-expected imaging depths, we successfully acquired over 7,200 fluorescence images. These images were free from crosstalk across three channels, facilitating large-scale, high-throughput imaging within just a few hours. This performance is largely due to the high-speed resonant scanning feature, which excels at efficiently capturing data from large sample areas.
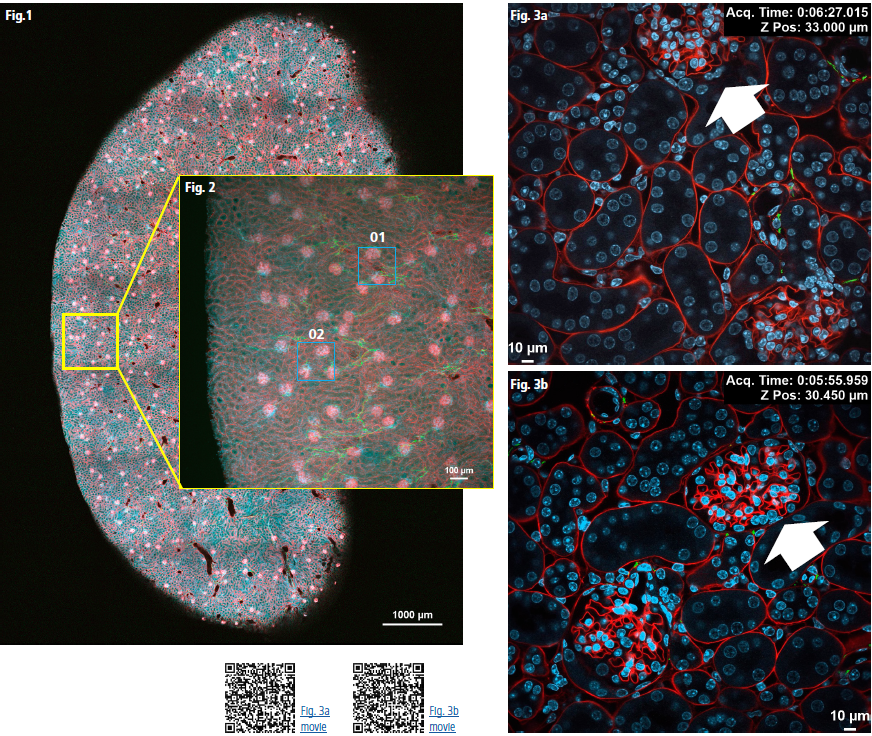
After identifying the target region using a low-magnification objective (Fig. 1, Fig. 2), we transitioned to an oil-immersion objective for detailed imaging. Z-stack acquisitions were performed rapidly with high-speed resonant scanning, ensuring both a broad overview and precise resolution of fine structures (Fig. 3a, 3b).
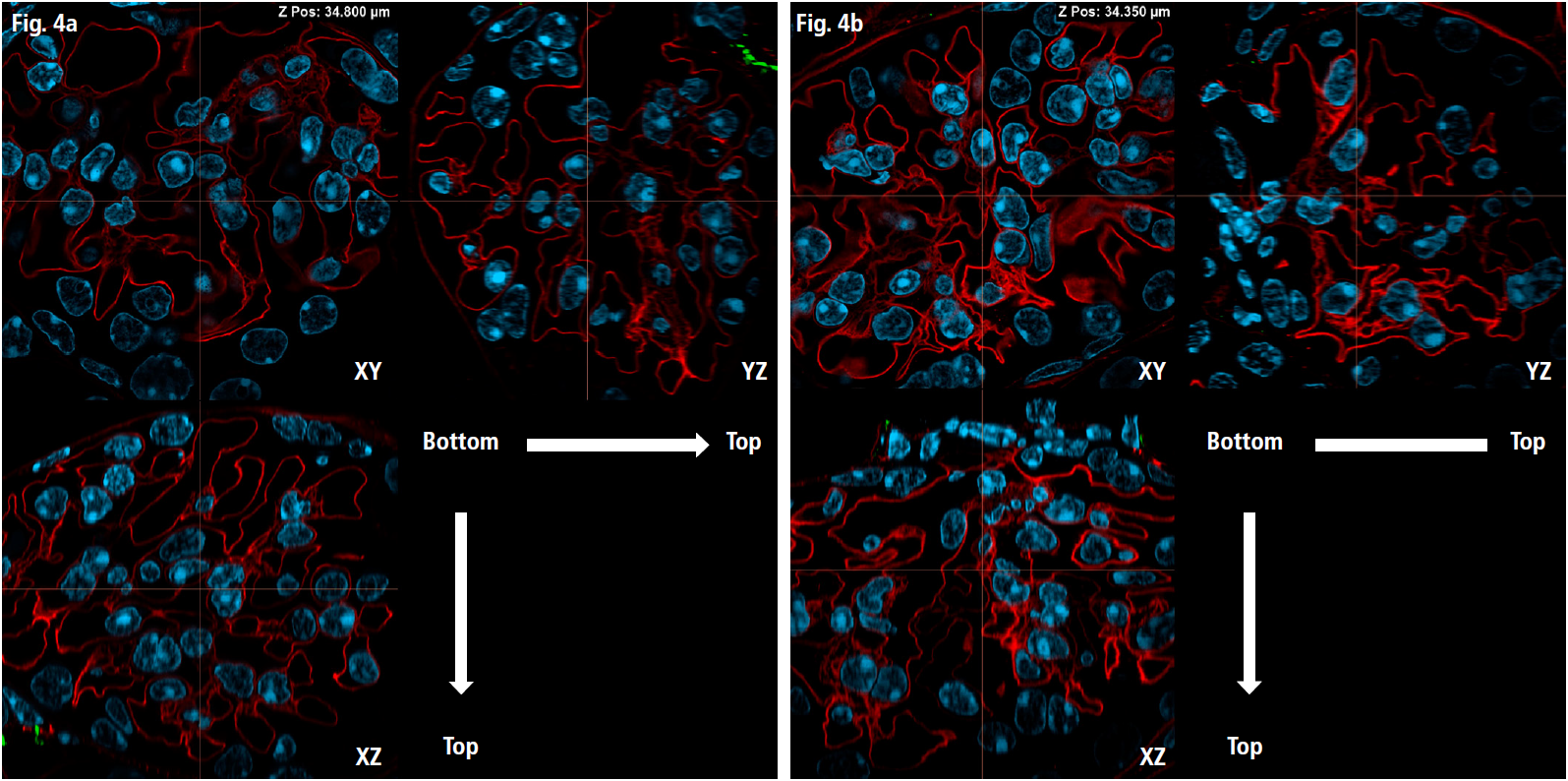
Finally, for super-resolution imaging of the intricate vascular network within the glomerulus, we utilized Image Scanning Microscopy (ISM) mode. This approach enabled the precise capture of detailed vascular structures located near the nuclei (Fig. 4a, 4b).
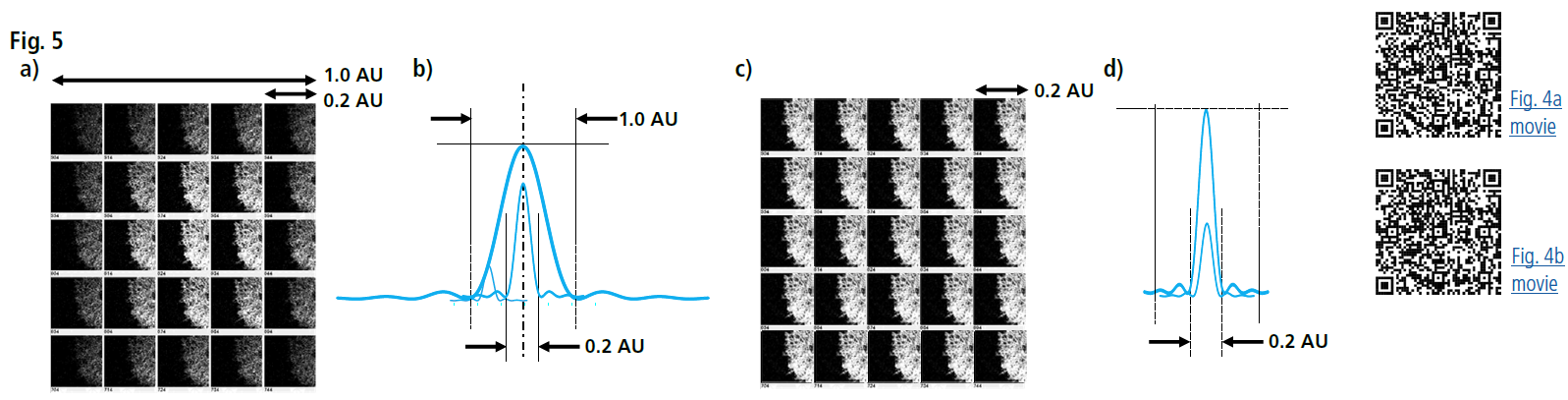
In ISM, the pinhole diameter was set to 0.2 Airy Units for signal detection (significantly smaller than the standard 1 Airy Unit). This setup, combined with pixel reassignment and deconvolution, improved resolution in the XY plane along the optical axis (Fig. 5).
Conclusion
Tomographic imaging, from the cover glass interface through the glomerulus to the opposite side, demonstrated consistent resolution along the optical axis. This stability is attributed to the uniform refractive index of the solid-state, polymer-embedded sample, which closely matches that of the immersion oil. As a result, optical aberrations were minimized, preserving high image quality.
Figure Legends
Fig 1: Global image of mouse kidney
DAPI-stained nudei (blue), cardiac perfusion with WGA-AF555 (red), and nerve fibers labeled with TUJ111-AF647 (green). The sample was embedded in Solid-SuperR polymer. Imaging was performed over a 5x7 stitched area with 206 z-stack slices, a z-step of 2.3 µm, and a z-travel range of 471 µm. Objective: Plan Apochromat Lambda D 10X/0.45, Total acquisition time: 4 hours 29 minutes, Image resolution: 1K x 1K pixels using resonant scanning, with a pixel size of 1.72 µm
Fig 2: Region of interest with areas 01 and 02 magnified in Figs. 3a and 3b, respectively
Imaging parameters: 1x1 region with 69 z-stack slices, a z-step of 2.3 µm, and a z-travel range of 156 µm, Objective: Plan Apochromat Lambda D 10X/0.45
Fig 3a: High-resolution image stack of area 01 in Fig 2
Imaging parameters: 458 z-stack slices, z-travel range of 68.4 µm, Acquisition time: 13 minutes 21 seconds, Image resolution: 1K x 1K pixels using resonant scanning, with a pixel size of 0.29 µm, Objective: Plan Apochromat Lambda D 60X Oil/1.42
Fig 3b: High-resolution image stack of area 02 in Fig 2
Imaging parameters: 514 z-stack slices, z-travel range of 76.8 µm, Acquisition time: 14 minutes 57 seconds
Fig 4a: Orthogonal images (XY, YZ, XZ planes) of the glomerulus (as indicated by the arrow in Fig. 3a), captured in ISM mode (NSPARC)
Imaging parameters: 469 z-stack slices, z-step of 0.15 µm, z-travel range of 70.05 µm, Imaging resolution: 1K x 1K pixels using resonant scanning, with a pixel size of 0.07 µm, Objective: Plan Apochromat Lambda D 60X Oil/1.42
Fig 4b: Orthogonal images of the glomerulus (as indicated by the arrow in Fig. 3b)
Imaging parameters: 496 z-stack slices, z-step of 0.15 µm, z-travel range of 74.1 µm, Imaging resolution: 1K x 1K pixels using resonant scanning, with a pixel size of 0.07 µm
Fig 5: Principles of Image Scanning Microscopy (ISM)
a) Image projections onto each channel of the detector array
b) Conceptual representation of the lateral Point Spread Function (PSF) from images on each channel. The final PSF and brightness after summation are comparable to a standard pinhole size of 1.0 Airy Unit.
c) Image projections after pixel reassignment.
d) Conceptual representation of the lateral PSF after pixel reassignment. The final PSF is comparable to a pinhole size of 0.2 Airy Units, resulting in enhanced lateral resolution without signal loss.
Acknowledgments and References
We would like to thank Prof. Shiue-Cheng (Tony) Tang at National Tsing Hua University, Taiwan, for preparing and providing the samples used in this study. The A-ha-based Solid-SuperR embedding technique utilized in this work is detailed in the following publication:
Transparent tissue in solid state for solvent-free and antifade 3D imaging,
https://www.nature.com/articles/s41467-023-39082-4
Similar samples can be obtained from Sunjin Lab Co. (Taiwan): https://www.sunjinlab.com/product-category/solid-superr
Product Information
AX/AX R with NSPARC Confocal-based Super Resolution Microscope
The NSPARC super-resolution detection module can be mounted to the AX/AXR Confocal Microscope. The 25 array detectors improve the resolution and S/N ratio, enabling high-speed and super-resolution imaging of complex tissue structures.
-
Nikon AX-R
-

Product information is here
스펙