Live-Cell Imaging of Plant Fertilization Using Nikon Multiphoton Microscope System
The mechanism by which a pollen tube (male) is guided to the ovule (female) within a flower is a type of chemotropism called pollen tube guidance. Crucially, only one pollen tube is guided, while subsequent pollen tubes are rejected. This phenomenon, known as polyspermy block, is thought to be a mechanism evolved to ensure successful fertilization and maximize seed production. However, the molecular mechanism of polyspermy block remains unclear, largely due to the difficulty of observing the internal structures of a living flower.
Dr. Yoko Mizuta and colleagues at the Institute of Transformative Bio-Molecules, Nagoya University, established a method for observing the internal structure of the Arabidopsis thaliana pistil using two-photon excitation microscopy, which offers high penetration depth and low invasiveness. By employing long-wavelength excitation (980 nm and above), optimized for deep imaging in plants, and fluorescent proteins suitable for two-photon excitation, they successfully captured the dynamics of pollen tube guidance and rejection in vivo (Mizuta et al., 2024). Image analysis revealed the spatiotemporal control of pollen tube behavior by both male and female gametophytes and maternal cells, uncovering a multi-step polyspermy block mechanism. This study sheds light on the sophisticated mechanisms employed by angiosperms to precisely control pollen tube dynamics within the flower, ensuring efficient reproduction.

Keywords: two-photon excitation, deep imaging, plant fertilization
Background and Aims
Traditional imaging of pistil tissues has been challenging due to their opacity and inherent autofluorescence. While conventional methods like dissection and chemical fixation provide structural insights, they compromise the observation of dynamic cellular processes and temporal relationships crucial for understanding mechanisms such as polyspermy block. To overcome these limitations, two-photon microscopy was explored for deep in vivo imaging. However, the pistil's complex structure, which scatters light, made internal observation difficult, and prolonged imaging, necessary for capturing polyspermy block, was challenging. To address these issues, authors of the study first identified optimal fluorescent proteins and excitation wavelengths for deep pistil imaging (Figure 1). Subsequently, a method suitable for live imaging of pollen tube growth and guidance deep within the pistil was established (Figure 2). This enabled the acquisition of detailed spatiotemporal information during the rapid processes of pollen tube attraction and rejection, with the ultimate goal of elucidating the molecular mechanisms underlying polyspermy block.
Figure 1. Background information
(a) Schematic diagram of a pollinated pistil. Pollen tube growth is thought to be influenced by both attractant and polyspermy block signals.
(b) Internal tissue structure of the pistil.
(c) Microscopy system used for observation (A1 R MP; current model: AX R MP).
(d) Comparison of two-photon excitation spectra of fluorescent proteins.
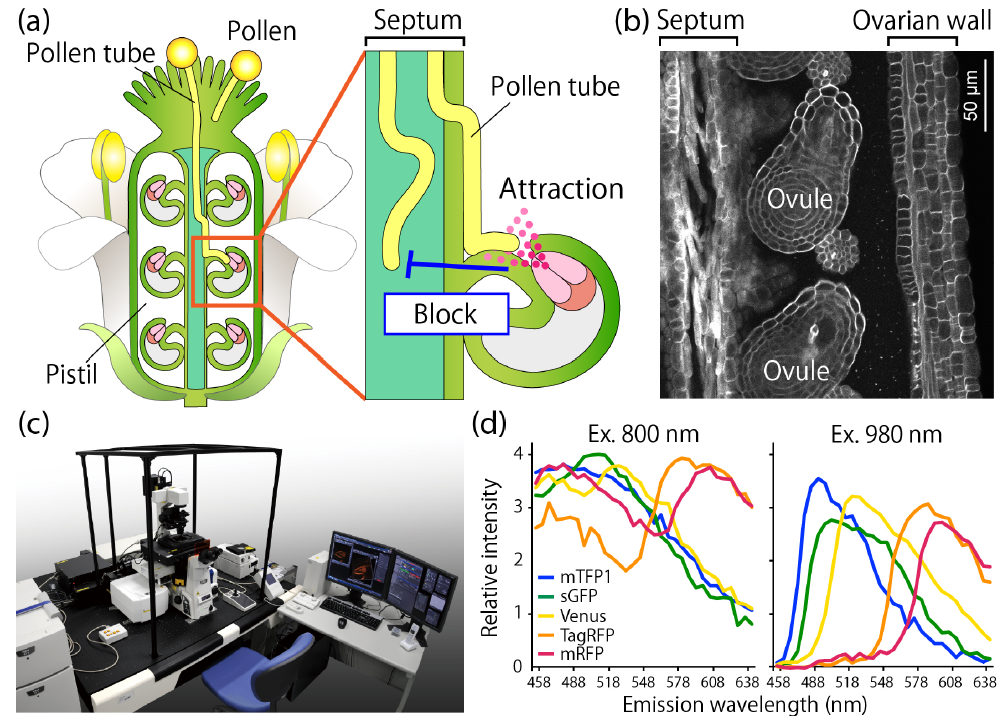
Figure 2. Overview of the Single-Locule Method
(a) Visualizing pollen tube growth deep within the pistil's transmitting tract requires specialized sample preparation techniques compatible with live-cell imaging. A single locule is carefully removed, and the pistil is mounted on a glass-bottom dish with the cut surface facing downwards onto a culture medium. To maintain sample stability and physiological conditions throughout extended imaging periods, authors developed a custom chamber system using a silicone sheet.
(b) The image shows five pistils arranged side-by-side, observed through the glass bottom of the dish after mounting.
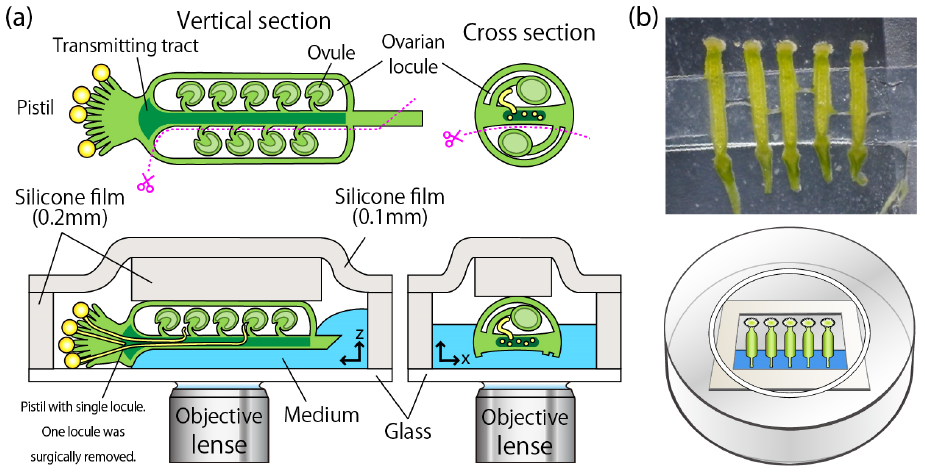
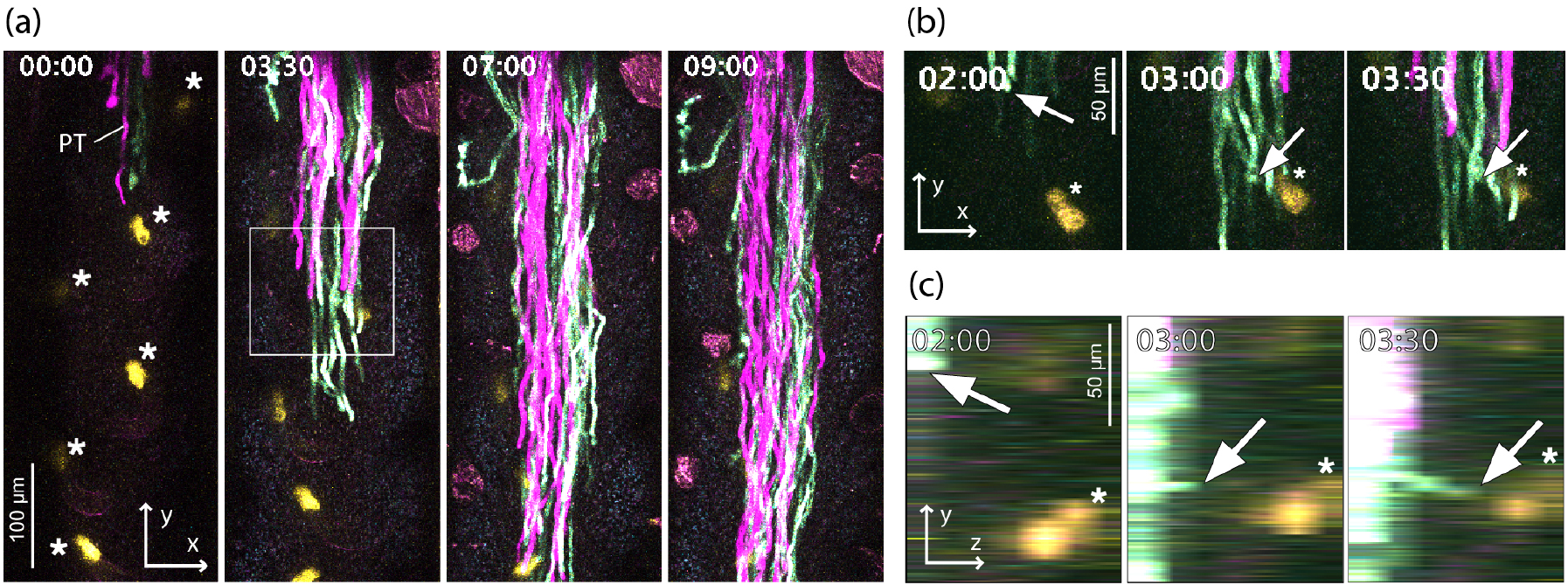
Figure 3. Live Imaging of Deep Pistil Tissues Using the Single-Locule Method
(a) xy image of a pollen tube growing inside the pistil. The pollen tubes are visualized in magenta and cyan, and the synergid cells of the ovule are shown in yellow. (b) Magnified view of (a). (c) yz view of (b). Arrows indicate the pollen tube guided toward the synergid cells. The asterisks mark the synergid cells. Time is indicated as hours:minutes. Modified from Mizuta et al. (2024).
Imaging conditions: Scan mode: galvano scanner, Resolution: 512 x 512 pixels, Objective lens: CFI75 Apo 25xW MP, WD = 2 mm; NA = 1.10
Establishing Deep Pistil Imaging and Key Findings
This study revealed that long-wavelength excitation (980-1000 nm) and orange fluorescent proteins are optimal for deep in vivo imaging of Arabidopsis pistils. Using a novel technique termed the "Single-locule method" (Figure 2), where one locule of the pistil is removed and the remaining pistil is mounted in a silicone chamber on a glass-bottom dish, the researchers successfully captured in vivo the dynamics of pollen tube guidance and polyspermy block (Figure 3) (Mizuta et al., 2024). Pollen tubes were observed to grow at an average speed of 1.3 µm/min, with a notable deceleration within the pistil's transmitting tract upon approach to the ovule. Analysis of mutants suggested that a long-distance signal originating from the maternal somatic tissue of the outer integument contributes to this deceleration.
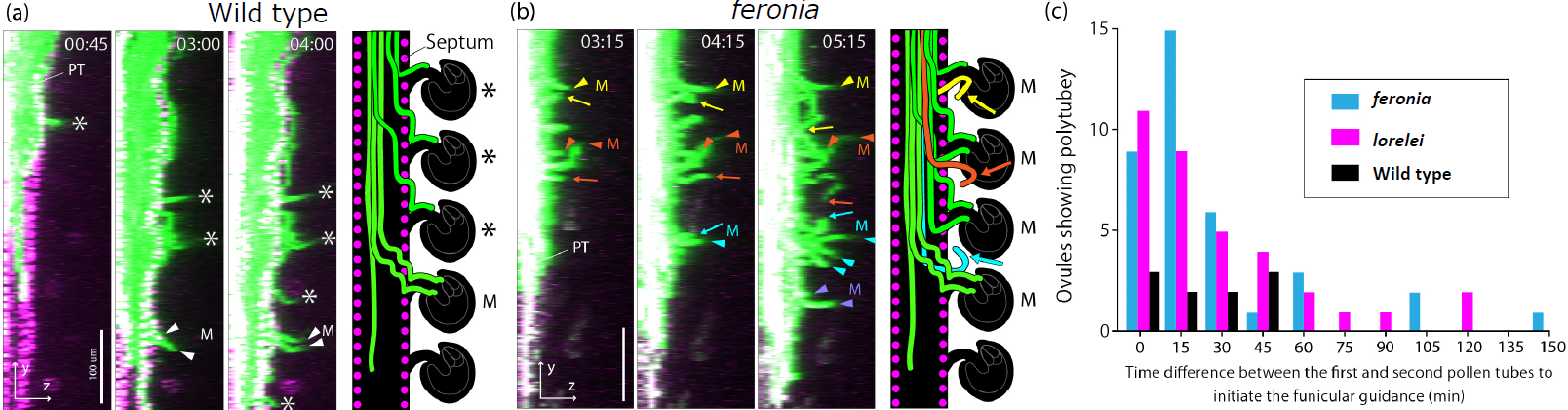
Furthermore, the study revealed a two-step polyspermy block mechanism, initiated earlier than previously thought, starting when the first pollen tube begins ascending the funiculus, which is then strengthened after 45 minutes. This two-step block involves FERONIA, a receptor-like kinase localized to the maternal cell membrane, and LORELEI, a GPI-anchored protein specifically expressed in synergid cells (Figure 4) (Mizuta et al., 2024). Intriguingly, in feronia mutants, a novel "repulsion" phenomenon was observed, where already-guided pollen tubes reversed their growth direction by 180°, seemingly attempting to return to the septum.
Figure 4. Live Imaging of Polyspermy Block in Wild-Type and Mutant Plants
(a) Wild-type. (b) feronia mutant. Pollen tubes are visualized in green, and nuclei of the septum epidermis in the pistil are shown in magenta. Asterisk (*) indicates ovules that attracted only one pollen tube; M indicates ovules that attracted multiple pollen tubes. In the mutant, almost all ovules attracted multiple pollen tubes (arrowheads). Arrows indicate pollen tubes exhibiting a "repulsion" phenotype, seemingly attempting to return to the septum after initial guidance. Time is indicated as hours:minutes. Scale bars: 100 µm. (c) Quantification of ovules attracting multiple pollen tubes and the timing of the second pollen tube attraction. Modified from Mizuta et al. (2024). Imaging conditions are the same as in Figure 3.
Conclusions
This study utilized two-photon microscopy to reveal the dynamics and signaling of pollen tube guidance within thick, autofluorescent plant tissues. The inherent advantages of two-photon microscopy, including reduced phototoxicity and deep tissue penetration, enabled clear visualization of the one-to-one guidance process between pollen tubes and ovules. The long working distance 25x water immersion objective facilitated stable deep imaging, accommodating tissue thickness and movement along the z-axis. Furthermore, the high-sensitivity GaAsP detector captured even weak signals from deep within the tissue, resulting in high-quality images. This study demonstrates the synergistic power of combining two-photon microscopy, a long working distance water immersion objective, and a highly sensitive detector for effective deep imaging.
Reference
Mizuta Y, Sakakibara D, Nagahara S, Kaneshiro I, Nagae TT, Kurihara D, Higashiyama T. Deep imaging reveals dynamics and signaling in one-to-one pollen tube guidance. EMBO Rep. 2024 Jun;25(6):2529-2549. doi:10.1038/s44319-024-00151-4.
Acknowledgment
We would like to express our sincere gratitude Dr. Yoko Mizuta of the Institute of Transformative Bio-Molecules (iTBM), Nagoya University, for her great cooperation and generous advice.
Edited by Shingo Nagawa, Nikon Corporation
Product Information
Multiphoton Microscope System AX R MP
The AX R MP is equipped with a high-speed resonant scanner with 2K resolution and can capture in a single scan dynamics that span a wide area with superior spatial and temporal resolution. Sensitivity is improved by utilizing the latest electrical and detector designs to drastically reduce noise that normally obscures dim signals. Detector configurations are flexible allowing the system to be tailored to any experimental needs.
스펙





